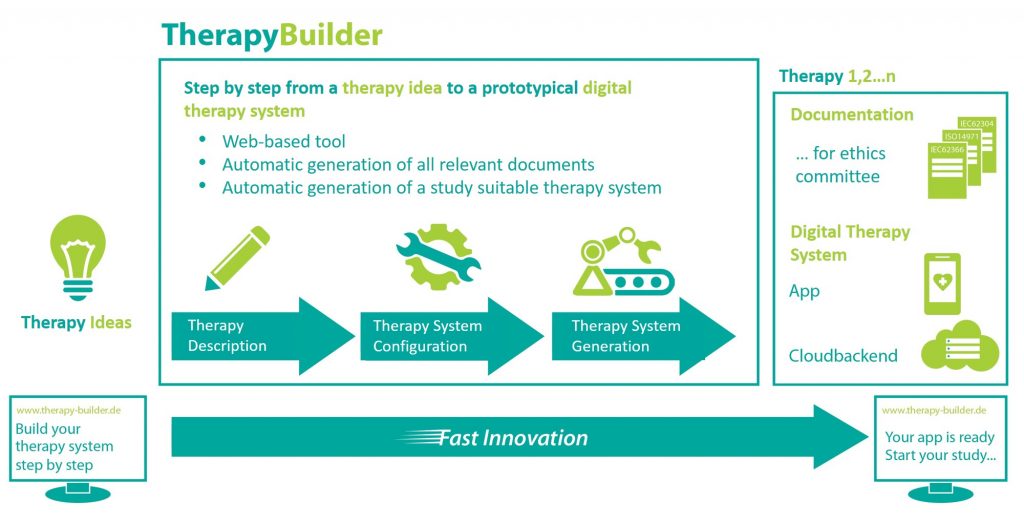
TherapyBuilder – Fast track from the idea to a mobile digital therapy system
Motivation
The rise of smartphone apps allows the realization of novel therapy methods for many diseases, enabling individual, location-independent and needs-based intervention. The challenge to date, lies in the fact that such therapeutic apps fall under the Medical Devices Regulation (MDR), requiring detailed knowledge of the complex regulatory processes and requirements of medical device development. This circumstance coupled with meagre resources and the sparse IT knowledge of medical experts mean that promising therapeutic approaches stay on the drawing board, and rarely become a product.
Goals and procedure
We're aiming to develop a web-based software tool that allows medical experts to create MDR compliant digital therapy systems - all without programming skills. The digital therapy systems consist of an app, including a cloud backend requiring users to spend only a few days with minimal financial investment in a language understandable to all participants. This allows the quickest way to evaluate the effectiveness of methods and therapy approaches in research studies.
To simplify compliance, we're developing a Regulatory Process Tool (RPT) to incorporate within the project. This RPT guides the user step by step through the complex regulatory processes and generates all relevant documents for submission to an ethics committee for the registration of a research study. To describe the actual therapy, we've developed a therapy modelling language (TML), with which the user can design the actual therapy for the TherapyBuilder to automatically generate a digital therapy system (app and cloud backend).
Innovations and perspectives
TherapyBuilder presents the first platform which generates prototypical, standards-compliant digital therapy systems in the shortest time and without in-depth IT knowledge. In contrast to previous solutions, these therapy systems may be used in clinical studies. TherapyBuilder both enables the evaluation of therapy approaches and methods with minimal cost and time and revolutionizes the speed of innovation for novel therapy approaches.
.
Project Coordination
movisens GmbH
Dr. Jürgen Stumpp
Augartenstraße 1, 76137 Karlsruhe
E-Mail: stumpp@movisens.com
Project Duration
01.09.2018 bis 30.08.2021
Project partners
- movisens GmbH, Karlsruhe
- FZI Forschungszentrum Informatik, Karlsruhe
- Karlsruher Institut für Technologie (KIT)
Project Management Agency
DLR PT-SW
Contact person: Michael Beichert
Tel.: (030) 67055-783
This project is funded by